
We will encounter these bonding situations in Chapter 5. Transition metal complexes containing halide ligands can also have significant pπ-dπ bonding, in which a filled pπ orbital on the ligand donates electron density to an unfilled metal dπ orbital. Source We are now going to explore some examples of sigma and pi bonds in the context of multiple bonds and identify how many sigma and pi bonds exist in double and triple bonds. The same kind of backbonding occurs with phosphine complexes, which have empty π orbitals, as shown at the right. The C-O infrared stretching frequency is diagnostic of the strength of the bond and can be used to estimate the degree to which electrons are transferred from the metal d-orbital to the CO π-antibonding orbital. This interaction strengthens the metal-carbon bond but weakens the carbon-oxygen bond. In metal carbonyl complexes such as Ni(CO) 4 and Mo(CO) 6, there is sideways overlap between filled metal d-orbitals and the empty π-antibonding orbitals (the LUMO) of the CO molecule, as shown in the figure below. Pπ-dπ bonding is also important in transition metal complexes. For example, phosphines (R 3P:) are good σ donors in complexes with transition metals, as shown below. Transition metal d-orbitals can also form σ bonds, typically with s-p hybrid orbitals of appropriate symmetry on ligands. com C2f6 Lewis Dot Structure 10 Images - Chem 111 Molecules Thei. Compounds with metal-metal δ bonds occur in the middle of the transition series. δ bonds are generally quite weak compared to σ and π bonds.
For this reason, compounds containing C=C double bonds are very common, but those with Si=Si bonds are rare. π-bonded compounds of heavier elements are rare because the larger cores of the atoms prevent good π-overlap. Because pπ-pπ bonding involves sideways overlap of p-orbitals, it is most commonly observed with second-row elements (C, N, O). In each case, we can make bonding or antibonding combinations, depending on the signs of the AO wavefunctions. Some possible σ (top row), π (bottom row), and δ bonding combinations (right) of s, p, and d orbitals are sketched below.

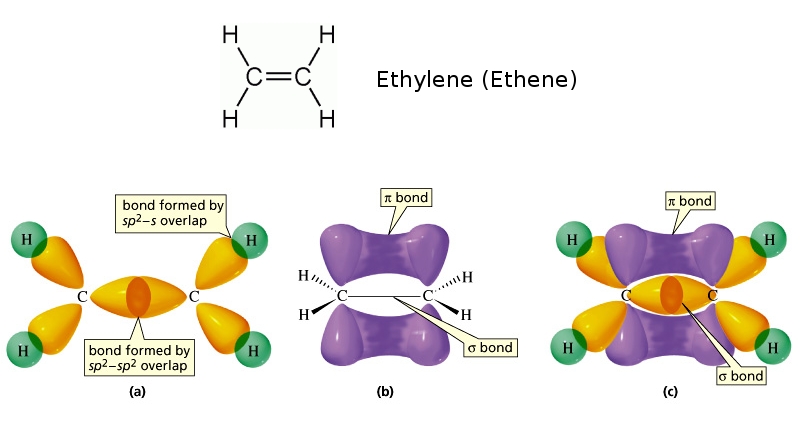
Thus in pi bond carbon carbon double bond rotation is restricted due to maximum overlap of p orbitals. Thus in any molecule in which pi bond formation takes place all the atoms must be in the same plane.
#C2f6 sigma and pi bonds free#
Free rotation of atoms around pi bonds is not possible because it involves breaking the pi bonds.\): The octachlorodirhenate(III) anion, 2−, which has a quadruple Re-Re bond. Pi () bond formation takes place by parallel orientation of the two p orbitals in adjacent atoms by proper sideways overlap.2. There can be free rotation of atoms around the sigma bonds.

In other words, a single bond cannot be a pi bond.

A total of two pi bonds and three sigma bonds for the acetylene molecule here. The sp of carbon 2 (YELLOW dumbbell) overlaps with the second hydrogens 1s (BLUE sphere) to make a mathbf(sigma) bond. There's our three sigma bonds and then we have a triple bond presence. Thus, a pi bond is always present in molecules with multiple bonds, i.e., double or triple bonds. Say that's a signal bond and then this bond over here we said was a sigma bond. The reason is that the atoms constituting a single bond prefer to form a strong sigma bond rather than a weak pi bond. The reason is that the overlapping of atomic orbitals can take place to a greater extent during the formation of a sigma bond, whereas overlapping of orbitals occurs to a smaller extent during the formation of a pi bond.Ī pi bond between two atoms is formed only in addition to a sigma bond. The orbital overlap takes place in such a way that their axes are parallel to each other but perpendicular to the internuclear axis.Ī sigma bond is stronger than a pi bond. This type of covalent bond is formed by the lateral or sideways overlap of the atomic orbitals. The atomic orbitals overlap along the inter-nuclear axis and involve end-to-end or head-on overlap. This type of covalent bond is formed by the axial overlapping of half-filled atomic orbitals.


 0 kommentar(er)
0 kommentar(er)
